Treatment Of Giardiasis
Giardia lamblia is both the most common intestinal parasite in the United States and a frequent cause of diarrheal illness throughout the world. In spite of its recognition as an important human pathogen, there have been relatively few agents used in therapy. This paper discusses each class of drugs used in treatment, along with their mechanism of action, in vitro and clinical efficacy, and side effects and contraindications. Recommendations are made for the preferred treatment in different clinical situations. The greatest clinical experience is with the nitroimidazole drugs, i.e., metronidazole, tinidazole, and ornidazole, which are highly effective. A 5- to 7-day course of metronidazole can be expected to cure over 90% of individuals, and a single dose of tinidazole or ornidazole will cure a similar number. Quinacrine, which is no longer produced in the United States, has excellent efficacy but may be poorly tolerated, especially in children. Furazolidone is an effective alternative but must be administered four times a day for 7 to 10 days. Paromomycin may be used during early pregnancy, because it is not systematically absorbed, but it is not always effective. Patients who have resistant infection can usually be cured by a prolonged course of treatment with a combination of a nitroimidazole with quinacrine.
Giardia lamblia, also called Giardia duodenalis or Giardia intestinalis, is a protozoan parasite of the small intestine that causes extensive morbidity worldwide. It was first described in the late 17th century by the Dutch microscopist Antonie van Leeuwenhoek62, and research into its epidemiology, pathogenesis, and treatment has intensified since G. lambliawaterborne outbreaks were reported in Europe and the United States during the 1960s and 1970s 53, 81, 123, 128, 174.Giardia infects approximately 2% of the adults and 6 to 8% of the children in developed countries worldwide and is currently responsible for the largest number of waterborne outbreaks of diarrhea in the United States 54, 139.
Despite the recognition of G. lamblia clinical illness for the last 40 years, the nearly 5,000 people hospitalized with giardiasis annually in the United States 149, and the millions infected worldwide, there have been few reviews of therapy for this infection and no definitive treatment protocols have been published58, 113, 150, 165, 261. In addition, only a handful of agents have been used in therapy, and the agents which are available may have adverse effects or be contraindicated in certain clinical situations. Also, resistance may play a role in some infections. This paper will review the agents currently used for the treatment of giardiasis. The history, mechanism of action, in vitro and clinical studies, and adverse effects are detailed for each drug class. In addition, special clinical situations are discussed and recommendations for therapy are made.
BACKGROUND
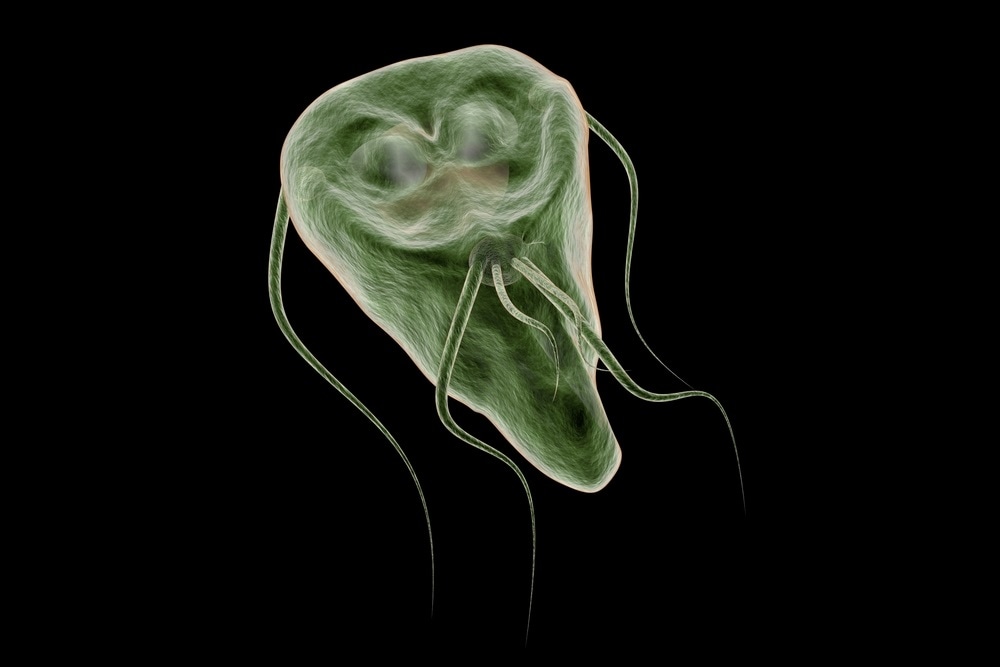
The life cycle of G. lamblia has two forms: the trophozoite (Fig. 1) and the cyst. The cyst is the infectious form and is ingested in contaminated water or food or directly from fecal-oral contact. As few as 10 cysts may establish infection 206. After ingestion, excystation occurs. Excystation is thought to be initiated by contact with acidic gastric contents, followed by a highly coordinated sequence of events leading to the release of one or two trophozoites 27, 109, 210. A parasite-derived protease may be activated during the excystation process 252. The trophozoite infects the duodenum and upper intestine, which have a favorable alkaline pH, and gives rise to the clinical sequelae. As trophozoites pass through the small intestine to the colon, encystation occurs. Encystation can be initiated in vitro by culture of parasites in a reduced concentration of bile salts and cholesterol followed by culture in an increased concentration of bile at an alkaline pH 156. Cyst wall proteins are then transcribed, secreted into encystment-specific vesicles, and transported to the newly forming cell wall over 14 to 16 h 75.
Fig. 1.
Ventral surface of a Giardia lambliatrophozoite imaged by scanning electron microscopy. It demonstrates the disk and flagella. A second trophozoite is seen behind it. Magnification, ×8,100. Photo courtesy of David Dorward, Rocky Mountain Laboratory, National Institutes of Health, Hamilton, Mont.
The wide variety of clinical presentations, from severe disease to an asymptomatic carrier state, makes the definitive determination of pathogenesis difficult. However, several theories have been put forward83, 115. Some of the most likely include the ability of the protozoan to cause direct damage to the intestinal mucosa via adherence with the disk, disaccharidase enzyme reduction following brush border damage, the release from Giardia of cytopathic substances such as thiol proteinases and lectins, and the stimulation of a host immune response with release of cytokines and mucosal inflammation 41, 48, 61, 78, 83, 106, 110, 157, 259. Additionally, it is likely that there are genetic differences betweenGiardia isolates which may confer virulence 115, 173, 182, 190. The surface of Giardia may also undergo antigenic variation in the human host and thus evade immune detection181. Given these multiple potential mechanisms, a multifactoral process is likely.
G. lamblia is found primarily in mammals including humans, cats, dogs, beavers, and cattle 40, 74, 77, 257. Transmission of the G. lamblia cyst to humans occurs most commonly following ingestion of contaminated water 139. Transmission via surface water is facilitated by the relative resistance of the cyst to chlorination and its ability to survive in cold water for weeks 59, 122. Transmission by food 22, 152, 187, by direct fecal-oral contact among children in day care 28, 224, 237 or in developing-world settings 96, 159, and by sexual practices which include oral-anal contact 168 represent other common modes of transmission 113. G. lamblia is also seen as a cause of prolonged diarrhea in travelers 37, 66, 125, 136, 205; N. Fiumara, Letter, N. Engl. J. Med.288:1410–1411, 1973).
Worldwide, the majority of patients infected with G. lamblia are asymptomatic. However, typical clinical symptoms of giardiasis usually begin 1 to 3 weeks after ingestion of cysts and are marked by diarrhea, malaise, flatulence, greasy stools, and abdominal cramps 113, 256. Other symptoms commonly include bloating, weight loss 174, and anorexia. Vomiting and fever are less common, and blood- or mucus-tinged feces are rare. Illness can last several months if untreated and can be characterized by continued exacerbations of diarrheal symptoms. With chronic illness, malabsorption of fat, lactose, vitamin A, and vitamin B12are reported, and failure of children to thrive has been noted86, 112, 149, 184, 227.
Giardiasis should be considered in the differential diagnosis of many diarrheal syndromes. A careful history, which notes any risk factors such as recent travel, wilderness exposure, or situations involving poor fecal-oral hygiene, and a physical examination are essential. Infection with G. lamblia can often be distinguished from bacterial and viral infections because of the longer duration of illness, 7 to 10 days by the time of presentation, and the presence of weight loss 113. Parasitic diarrhea withCryptosporidium or Cyclospora can have similar features in the immunologically normal host and would need to be distinguished by specific diagnostic testing 50, 99.
Several methods exist for detection of the parasite. Demonstration of trophozoites or cysts in the stool, called the ova and parasite (O&P) examination, is the traditional means of diagnosis 167. One stool sample will allow the detection of 60 to 80% of infections, 2 stool samples will allow the detection of 80 to 90%, and three stool samples will allow the detection of over 90% 97, 111. However, in some instances, because of intermittent or low levels of shedding, it is necessary to examine more than three stool samples. The desire for more sensitive and specific, as well as faster and reproducible, diagnostic testing has led to the development of immunoassays. Fecal antigen detection using enzyme-linked immunosorbent assays, nonenzymatic immunoassays, or fluorescein-tagged monoclonal antibodies can be superior diagnostic methods to the O&P examination7, 89, 90, 161, 262. It is particularly helpful in assessing cure or in screening for Giardia infection. However, when other parasites are in the differential diagnosis, a stool sample for O&P examination should still be ordered. In the unusual patient for whom a diagnosis cannot be made by O&P examination of stool, endoscopy with duodenal fluid sampling and biopsy may be performed 82, 113, 185, 256. An instance in which this may be helpful is the human immunodeficiency virus-infected patient with diarrhea, whose illness has multiple potential etiologies. Culture and sensitivity testing is available only in research settings 120, 138. DNA probes have been generally limited to detection of parasites in water samples 134, 158, and serologic testing is most useful in epidemiologic surveys 118, 119, 170. Figure 2 outlines an approach to the diagnosis and management of suspected cases of giardiasis and is discussed further below (see “Recommendations”).
Fig. 2.
Outline of the diagnosis and management of suspected cases of giardiasis.
THERAPY OF GIARDIASIS
When evaluating the clinical efficacy of agents used againstGiardia, it is difficult to compare studies. They vary as to entry methodology (whether randomization was done and if treatment was blinded or open), population studied (children, adults, symptomatic and/or asymptomatic patients), outcome measures (clinical efficacy and/or stool negativity), and duration of follow-up. Nevertheless, conclusions may be drawn from the studies when viewed as a whole, and statements can be made about the relative efficacy of the agents.
Classes of Agents and Clinical Properties
Nitroimidazoles.The nitroimidazoles class of agents used to treat G. lamblia infection includes metronidazole, tinidazole, ornidazole, and secnidazole. This class was discovered in 1955 and was found to be highly effective against several protozoan infections 240. Metronidazole [1-(β-hydroxyethyl)-2-methyl-5-nitroimidazole; Flagyl] was determined to be therapeutic against Trichomonas vaginalisand Entamoeba histolytica following its discovery in the late 1950s 67, and in 1962 Darbon et al. reported that it could be used to treat giardiasis 57. Since this discovery, metronidazole and other nitroimidazoles have been used by clinicians as the mainstay of therapy of giardiasis.
Of the nitroimidazoles, the mechanism of killing of Giardiaby metronidazole has been the most thoroughly studied. Metronidazole utilizes the anaerobic metabolic pathways present inGiardia. The drug enters the trophozoite, and once it is within the cell, electron transport protein ferredoxins from the parasite donate electrons to the nitro group of the drug 223, 238, 244. The drug becomes “activated” by reduction of this nitro group 223, 238, 240, and a gradient favoring the intracellular transport of metronidazole is established by this reduction reaction. Reduced metronidazole serves as a terminal electron acceptor which binds covalently to DNA macromolecules 72, 177. This results in DNA damage in the form of loss of helical structure, impaired template function, and strand breakage, with subsequent trophozoite death 95. In addition to this effect, metronidazole inhibits trophozoite respiration 81, 189. The reductive activation of metronidazole may also lead to toxic radicals, which react with essential cellular components244. Trophozoites within cysts may be less affected by nitroimidazoles, possibly because of poor penetration of drug through the cyst wall 236. Resistance to metronidazole has been induced in vitro 29. It correlates with decreased activity of parasite pyruvate:ferredoxin oxidoreductase, which is required for reductive activation of nitroimidazoles 239, 247.
Metronidazole is quickly and completely absorbed after oral administration and penetrates body tissues and secretions such as saliva, breast milk, semen, and vaginal secretions 240. The drug is metabolized mainly in the liver and is excreted in the urine 147.
In vitro assays for nitroimidazole drug susceptibility have been performed with G. lamblia since 1980112. Using microscopic evaluation of parasite morphology and mobility, Jokipii and Jokipii first demonstrated that metronidazole and tinidazole were effective 129. Subsequently, morphology 13, 166, 175, growth inhibition 56, 69, 94, 116, 160, 225, [3H]thymidine incorporation32, 117, 164, serum killing 114, vital-dye exclusion 114, 235, inhibition of adherence 21, 55, 79, 166, 192, metabolic 228, and colorimetric133 assays have been employed to measure the in vitro response of the drug to many therapeutic agents. However, as indicated by the variety of assays used, there is no standard for in vitro testing, making it difficult to compare results and apply in vitro findings to the clinical setting.
Of the nitroimidazoles, tinidazole and metronidazole have consistently demonstrated the greatest in vitro activity; tinidazole possesses a slight advantage 30, 32, 55, 101. More highly substituted nitroimidazoles, such as miconazole, clotrimazole, itraconazole, and ketoconazole, were developed for their antifungal activity and are not effective agents against G. lamblia55. Sensitivity to nitroimidazoles can vary depending on the stocks and clones of G. lamblia used in testing 29, 31, 79, 160.
In the United States, metronidazole is the only member of the nitroimidazole class available to treat giardiasis; it is also the most common drug used for treatment worldwide. In spite of its widespread and accepted use against Giardia, the U.S. Food and Drug Administration has never approved it for this indication. Clinical trials have employed dosing two and three times daily (usually 250 mg/dose) for 5 to 10 days and short-course (1 to 3 days), daily single-dose therapy (2.0 or 2.4 g/dose) 261. In the 5- to 10-day schedules the efficacy ranges from 60 to 100% in adult and pediatric patients, with a median efficacy in both groups of 92% (Table 1) 20, 49, 68, 91, 92, 100, 127, 132, 135, 150, 151, 191, 201, 202, 232, 256. In general, this schedule is well tolerated, with most side effects involving gastrointestinal upset and metallic taste (Table2).











 Play Store
Play Store
Comments (0)
No Comments.